
Powered by our Glyph platform we are advancing first and best-in-class medicines for people living with neuropsychiatric disorders.
Powered by our Glyph platform we are advancing first and best-in-class medicines for people living with neuropsychiatric disorders.
We have a proven strategy of advancing clinically validated mechanisms — previously held back by limitations — which our technology platform can now overcome.
GLYPH™ BENEFIT
DISCOVERY
PRECLINICAL
PHASE 1
PHASE 2
GlyphAllo™ (SPT-300)
Glyph Allopregnanolone
GLYPH™ BENEFIT
Overcome lack of oral bioavailability
GlyphAgo™ (SPT-320)
Glyph Agomelatine
GLYPH™ BENEFIT
Negate need for liver function testing
Glyph2BLSD™ (SPT-348)
Glyph 2-bromo-LSD
GLYPH™ BENEFIT
Improve PK & tolerability
Additional Programs
GLYPH™ BENEFIT
Overcome lack of bioavailability, improve PK & tolerability
Completed
In-progress
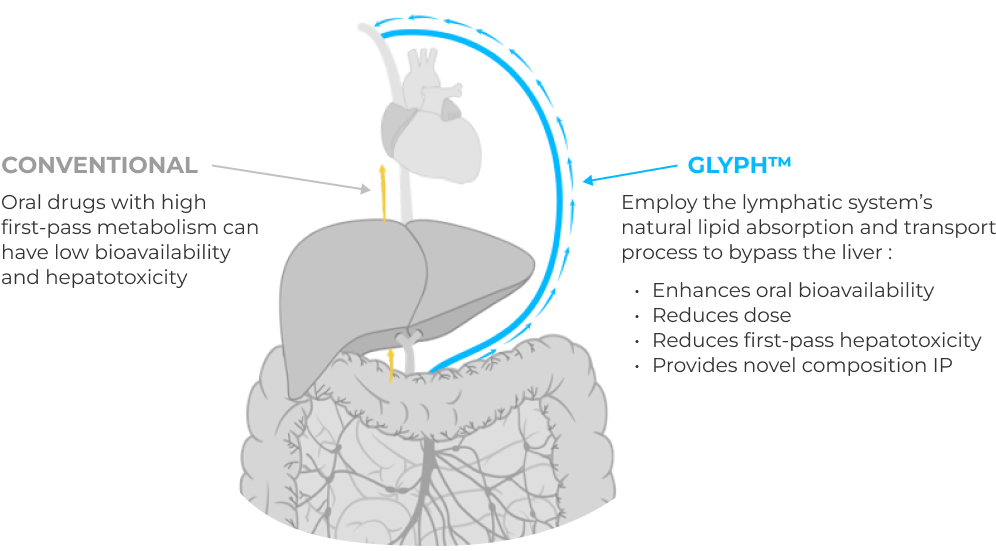
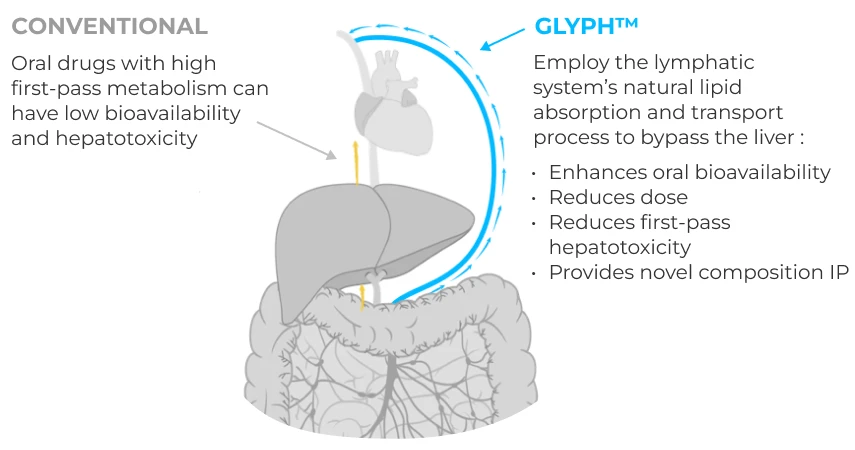
Glyph platform unlocks potential of drugs across CNS and non-CNS therapeutic areas
1 The FDA and corresponding regulatory authorities will ultimately review our clinical results and determine whether our therapeutic candidates are safe and effective.
No regulatory agency has made any such determination that our therapeutics are safe or effective for use by the general public for any indication.



Proprietary platform advances active drugs previously limited by low oral bioavailability/hepatotoxicity.
Glyph™ is based on the pioneering research of the Porter research group at Monash University in Melbourne.

Christopher Porter, Ph.D.
Original Co-inventor of Glyph Technology, Director of the Monash Institute of Pharmaceutical Sciences
The group and it’s collaborators have published research in multiple publications supporting the Glyph™ platform’s capabilities.
Co-inventors of the breakthrough technology include members of the Seaport and PureTech teams.

Jamie Simpson, Ph.D.
Original Co-inventor of Glyph Technology, Head of Chemistry at Seaport
Dan Bonner, Ph.D.
Co-founder, Senior Vice President, Platform at Seaport

Cobenfy™ (formerly known as KarXT), which was invented and developed by members of our team, has demonstrated notable improvements across schizophrenia symptoms – without the debilitating side effects of existing drugs – and is now poised to be the first new class of medicine in over 50 years for patients living with schizophrenia. Karuna was acquired by Bristol Myers Squibb for $14B in March 2024 and received FDA approval in September of 2024.
Patient Need
Schizophrenia is a chronic psychiatric condition affecting 21M+ people globally. Up to 74% of patients discontinue medication before 18 months, with many failing to find an effective and/or tolerable therapy. Patients are in need of medicines that work differently than the current standards of care, which can cause weight gain, sedation and movement disorders.
Validated Efficacy
Xanomeline had generated exciting efficacy data at Eli Lilly, under the leadership of Steve Paul, but the drug was not advanced due to tolerability issues.
Our Team's Innovation
To address this challenge, Cobenfy (formerly KarXT) was developed by combining xanomeline (a muscarinic receptor agonist) with trospium (a peripherally acting antagonist that does not cross the blood–brain barrier). This breakthrough unlocked the development of the first new class of medicine for schizophrenia in over 50 years. Cobenfy originated at PureTech, which was led by Daphne Zohar and where Karuna was founded, and Dr. Paul who joined Karuna as CEO. Under Dr. Paul’s leadership, Karuna advanced the program through clinical development, culminating in three successful pivotal studies and FDA approval.
Cobenfy™ (formerly known as KarXT), which was invented and developed by members of our team, has demonstrated notable improvements across schizophrenia symptoms – without the debilitating side effects of existing drugs – and is now poised to be the first new class of medicine in over 50 years for patients living with schizophrenia. KarXT was named the most anticipated drug launch of 2024 by Evaluate Vantage.
Karuna was acquired by Bristol Myers Squibb for $14B in March 2024.
Patient Need
Schizophrenia is a chronic psychiatric condition affecting 21M+ people globally. Up to 74% of patients discontinue medication before 18 months, with many failing to find an effective and/or tolerable therapy. Patients are in need of medicines that work differently than the current standards of care, which can cause weight gain, sedation and movement disorders.
Validated Efficacy
Xanomeline had generated exciting efficacy data at Eli Lilly, under the leadership of Steve Paul, but the drug was not advanced due to tolerability issues.
Our Team's Innovation
To overcome this, Cobenfy (formerly known as KarXT) was invented by combining xanomeline (a muscarinic receptor agonist) with trospium (a peripherally acting antagonist that doesn’t cross the blood brain barrier), and in doing so, our team unlocked development of the first new class of medicine for schizophrenia in over 50 years. This new medicine was invented at PureTech and advanced by Karuna, which was co-founded and led by members of our team.
